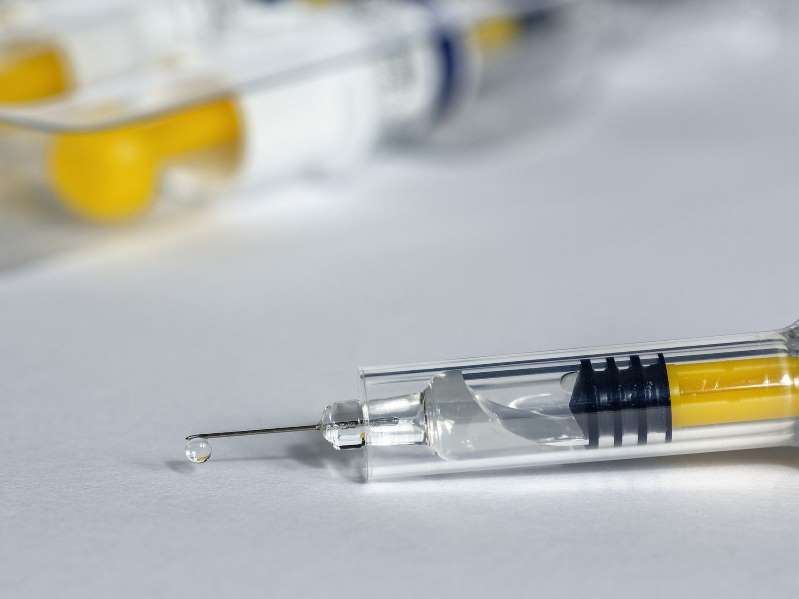
There are currently more than 150 different COVID-19 vaccines under development. Of these, more than 50 have reached human trials, and several have been approved for emergency or full use on six continents. In the US, two vaccines—one from the biotechnology company, Moderna and the other from drug giant Pfizer—have been granted an Emergency Use Authorization (EUA) from the FDA. To read the full story.
Recent Posts
- To Heal Skin, Scientists Invent Living Bioelectronics.
- Register for Children’s Specialized Hospital Distinguished Lecture on 8/14
- Researchers Shed Light on Cause of ‘Happy Hypoxia’ in COVID-19 Patients.
- Upending Conventional Wisdom, Cannabis Use Doesn’t Hinder PTSD Therapy.
- Many Firearm Owners Can’t Recognize When a Cable Lock Is Properly Installed.
Categories
- News (2,131)
- Publication (1)