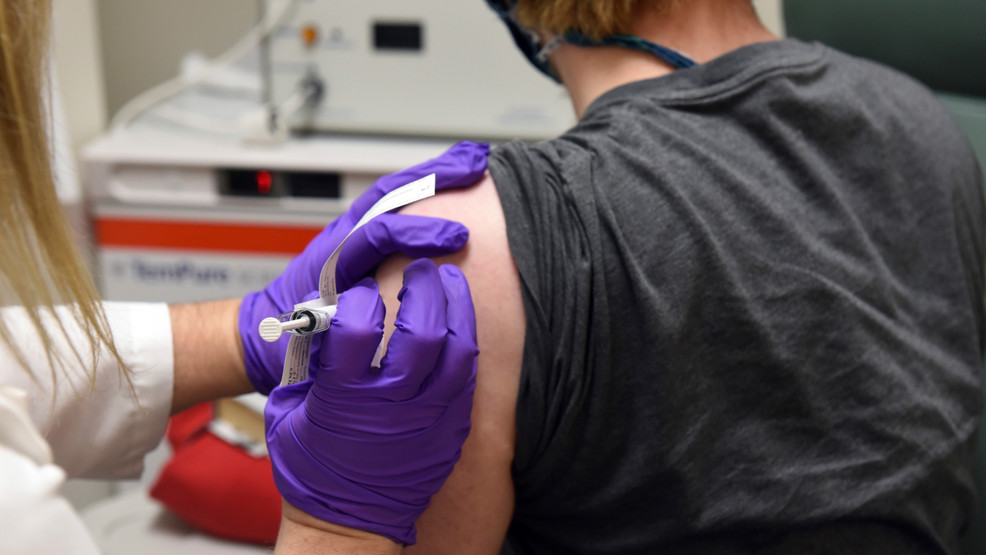
With coronavirus cases surging in the United States, the need for a vaccine is growing increasingly urgent, but federal officials have sought in recent days to reassure the public that the approval process for any potential vaccine will be thorough and transparent. Pfizer and BioNTech announced Wednesday that the latest data from their vaccine study indicates their shot is 95% effective, and they intend to formally apply for emergency authorization (EUA) in the U.S. within days. The companies claimed no serious side effects had been detected, and the most common problem was fatigue reported by 4% of participants. To read the full story.
Recent Posts
- To Heal Skin, Scientists Invent Living Bioelectronics.
- Register for Children’s Specialized Hospital Distinguished Lecture on 8/14
- Researchers Shed Light on Cause of ‘Happy Hypoxia’ in COVID-19 Patients.
- Upending Conventional Wisdom, Cannabis Use Doesn’t Hinder PTSD Therapy.
- Many Firearm Owners Can’t Recognize When a Cable Lock Is Properly Installed.
Categories
- News (2,131)
- Publication (1)