RITMS Center for Cellular Dynamics & Physics of Living Cells
This Center focuses on understanding the mechanobiology in health and diseases. We develop and apply state-of-the-art technologies to modulate and visualize the physical and molecular forces in living cells that regulate how cells and tissues grow, contract, move, invade, and remodel. Understanding the underlying mechanisms of these fundamental biological processes can provide new approaches to tissue regeneration and therapy for diseases such as asthma and cancer.
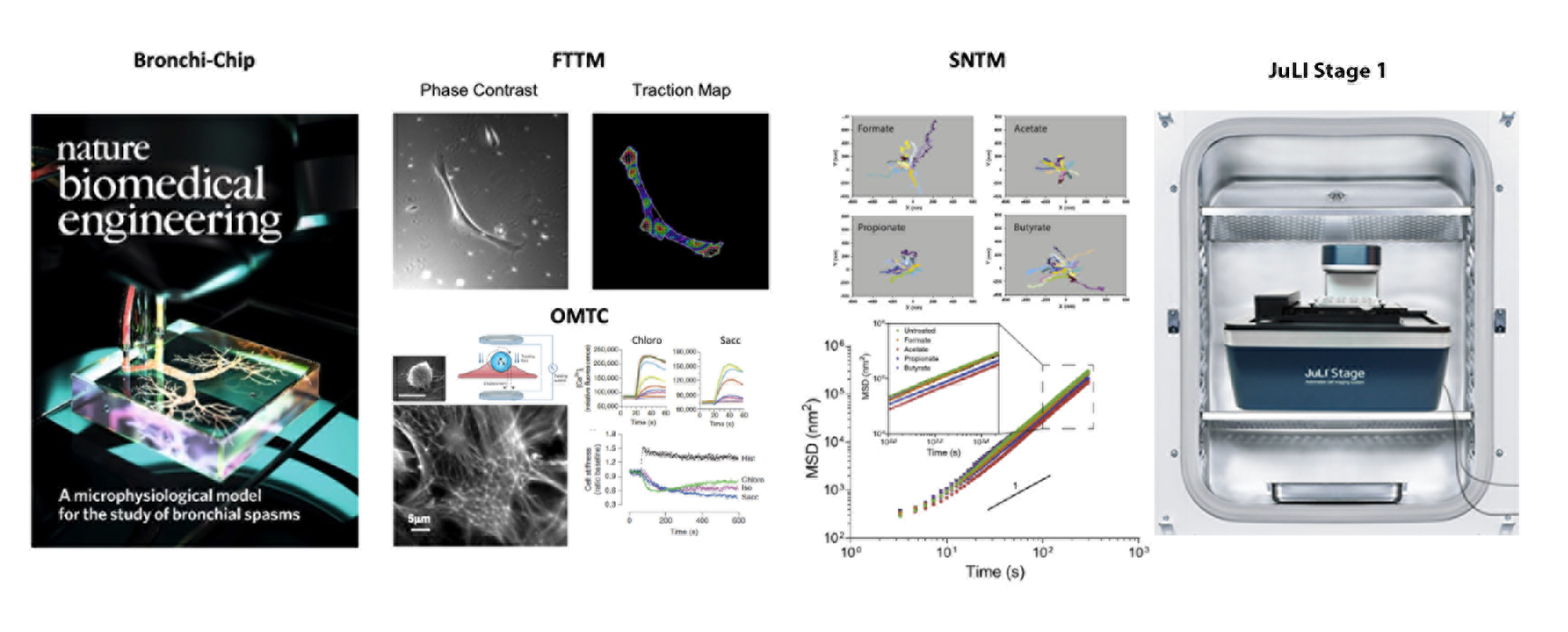
The Center has the unique capability to map the cellular stress using Fourier transform traction microscopy (FTTM), to probe the material properties of living cytoskeleton (CSK) using optical magnetic twisting cytometry (OMTC), and to quantitate the molecular-level remodeling of internal structures using spontaneous nanoscale tracer motions (SNTM). Our live-cell micromechanical methods are readily applicable to a wide variety of cell types and have broad research applications that are at the interface between engineering, cell biology and medicine.
Applying these novel imaging methods, for example, we have uncovered an entirely new chemo-mechanical signaling network of ‘sensory’ G protein-coupled receptors of the bitter taste receptor (TAS2R) family and the olfactory receptor (OR) family expressed on the smooth muscle of human bronchi. We have ascertained that agents that activate TAS2Rs on human airway smooth muscle (ASM) cells are effective bronchodilators, showing a favorable therapeutic profile against asthmatic bronchospasm or other obstructive lung diseases. In the same vein, we have shown that activation of the odorant receptor OR51E2 expressed on human ASM cells via its cognate ligand acetate and propionate inhibits cytoskeletal remodeling and cellular proliferation. These physiologic outcomes mediated by endogenous metabolic byproducts of the gut microbiota (i.e. short chain fatty acids) suggest previously unidentified “ancient” chemosensors of the gut-lung axis. The findings also give rise to the notion that ectopic expressions of sensory receptors can be exploited to discover novel disease-modifying therapeutics for asthma, which would represent a clear shift in asthma treatment paradigms.
Current research efforts include: 1) identifying molecular machines primed for sensation in the lung; 2) repurposing the double helix to probe, in real-time, excitation-contraction coupling in smooth muscle shortening; 3) developing ‘bronchi-chip’ that enables chemical and mechanical interrogation into heterotypic cell-to-cell communications; 4) defining the physics of cancer cell migration and invasion.
 We welcome inquiries, consultations, and collaborations. To contact:
We welcome inquiries, consultations, and collaborations. To contact:
Steven An, Ph.D., Director of Bioengineering, Rutgers Institute for Translational Medicine & Science
732-235-9132 or sa1510@rbhs.rutgers.edu